
Since 2008, Vietnam has been classified as a pharmerging country, according to IMS Health (IQVIA). Pharmaceutical spending per capita in Viet Nam is relatively low but has increased dramatically in recent years. In 2005: 9,85 USD (equivalent to about 227.000 VND), in 2014: 34,48 USD (equivalent to about 793.000 VND), forecasted to reach to 163 USD by 2025 (equivalent to about 3.749.000 VND). At the same time, the large population and rapid aging in Vietnam have promoted high demand for health care and especially the demand for pharmaceutical products (the proportion of elderly people in the period 1979 – 2015 accounted for 6,9% to 11,3%, and Vietnam will exceed 25% of the total population by 2049).
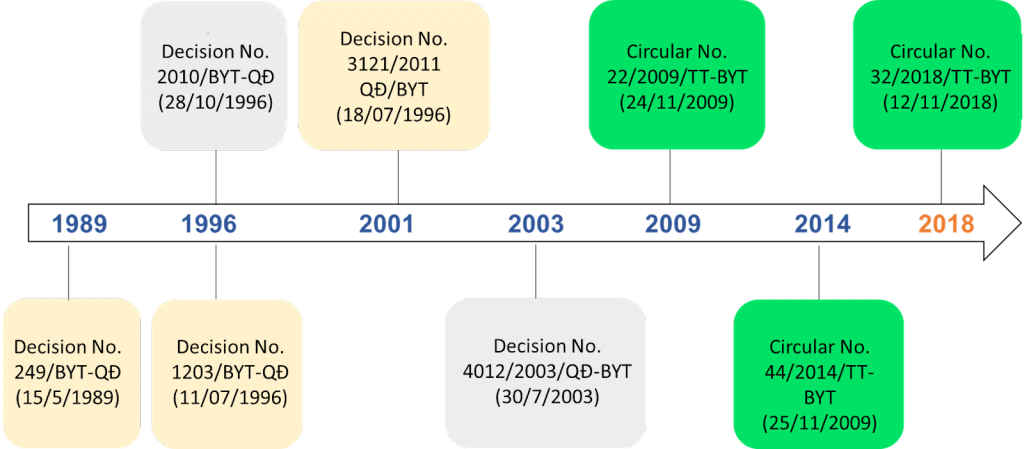
Drugs are a special commodity that directly affects the health of the users. Therefore, drugs that want to be circulated on the market and distributed to users must be issued with a registration number by the Ministry of Health (MOH), specifically the Drug Administration Department. The registration of drugs is a mandatory legal barrier to minimizing the occurrence of counterfeit drugs and poor-quality drugs in the market. In the national strategy for the development of Vietnam’s pharmaceutical industry up to 2020 with a vision toward 2030, the development viewpoint is adequately supplied preventive and curative drugs for people with ensured quality, and rational prices; build the pharmaceutical industry, which concentrates on the development investment to produce the generic drug with ensured quality, rational prices, gradually replace import medicines, Regulations on drug registration in Vietnam have passed 31 years since the first Decision No. 249/BYT-QD was issued on May 15, 1989. Accordingly, there have been many changes to improve the regulations on drug registration in Vietnam. Circular 22/2009/TT-BYT marked a significant change in the regulations on drug registration when consolidating the registration of modern medicines, traditional drugs, herbal drugs, vaccines, and biologicals. Decisions and circulars regulating the registration of drugs have been issued in Vietnam from 1989 to now are shown in the diagram below.
In the period of 11 years, from 2009 to 2019, Vietnam had 45.628 drug registration numbers (RNo).
Two milestones are 2010 (Circular 22/2009/TT-BYT in force) and 2015 (Circular 44/2014/TT-BYT in force).
From 2010 onwards, the number of RNo has a strong downward trend, of which 1.890 in 2011 (26,5% less than in 2010) and 3.873 in 2012 (45% less than in 2010).
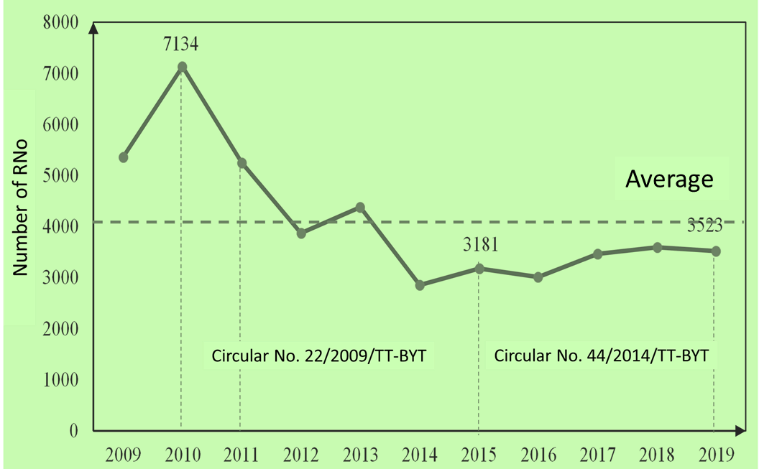
The advent of Circular 22/2009/TT-BYT strongly impacted drug registration in our country, which is the first circular that provides for the general registration of modern medicines, traditional drugs, herbal drugs, and vaccines and biologicals in the same circular. The change in the regulations on drug registration dossiers of Circular 22 compared to the previous Decision 3121/2001/QD-BYT had adjusted the classification of drug registration dossiers according to the origin of manufacture to classification by drug type. The regulation of drug registration is more closely controlled in Circular 22 and is the first milestone marking the adoption of the ASEAN Common Technical Document (ACTD) in drug registration dossiers.
Regarding the origin of drugs, the number of RNo is 28.219, accounting for 61,8% of the total number of RNo; for foreign drugs, the average number of 11 years accounts for 38,2% (17.409 RNo).
In recent years, foreign drugs have tended to decrease both in terms of the number of RNo and the percentage, specifically since 2016 – 2019, the number of RNo between 813 – 1012 (accounting for less than 50% compared to the number of RNo in 2009 – 2011) and the percentage of foreign drugs always accounted for less than 30% of the total RNo.
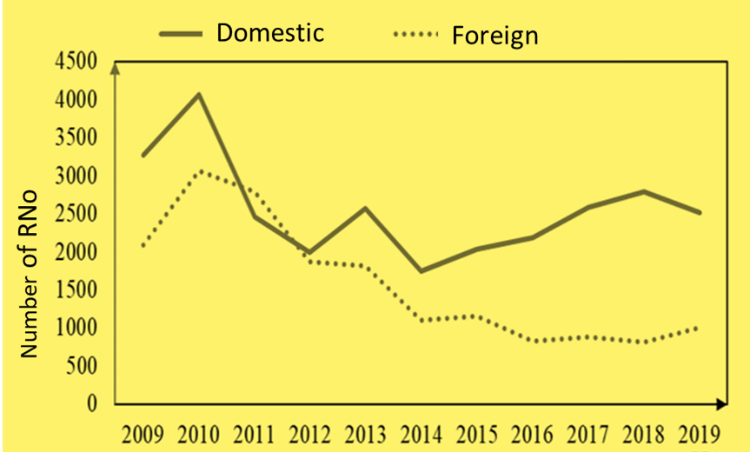
The increase in the percentage of domestic drugs compared to foreign drugs may result from the policies of support, priority, and encouragement of the State management agency for domestic pharmaceutical enterprises in increasing the production, research, registration of drugs as well as priority in the procurement of drugs to respond to treatment demand of the health facility. This is reflected in the Law on Pharmacy No. 105/2016/QH13: “Priority shall be given to purchasing of generic drugs and biosimilars that are domestically produced and granted certificates of free sale in Vietnam; herbal drugs, traditional drugs derived from domestic herbal ingredients.” In addition, the priority of using domestically produced drugs enterprises is also reflected in the project “Vietnamese people give priority to using Vietnamese drugs,” approved by the Ministry of Health in Decision No. 4824/QD-BYT issued on December 3, 2012. These policies have contributed to the motivation of domestic drug companies to strengthen drug research and development to reduce their dependence on imported drugs.
In 2001, only about 36 countries with RNo were granted; in 2009 – 2019, more than 50 countries with registered medicines distributed each year (on average, 52 countries/year).
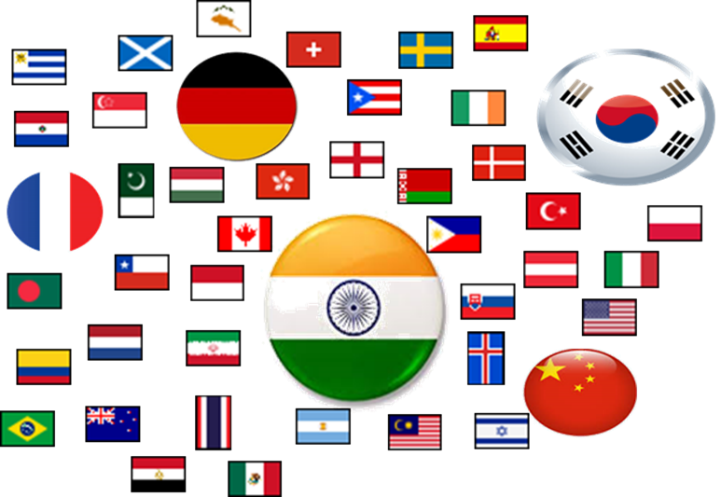
The increase in the number of countries registering drugs shows that the Vietnamese pharmaceutical market is increasingly confirmed as a potential market, attracting much attention from companies and businesses worldwide. Although the number of registered countries has increased by about 1,3 times compared to 2001, most registered foreign drugs originate from India (accounting for 33,9%) and Korea (accounting for 17,3%). In the period of 2009 – 2019, and the period of 2000 – 2006, they were the two countries with the most RNo when accounting for 31,5% and 21,2%, respectively. It can be seen that India and Korea account for more than 50% of the RNo granted, while some such countries have very few RNo for the whole 11-year period, such as Croatia (2 RNo), Lithuania (2 RNo); Slovakia (4 RNo) this indicates an imbalance in the structure of countries registering drugs in Vietnam. However, in recent years, the number of RNo and the percentage of total RNo issued by the ten countries, including India and South Korea, are on a declining trend. Specifically, India, from 2015 – to 2019, has always accounted for less than 30%, and the percentage of 10 countries has also accounted for less than 70%; it can be seen that there has been an increase in the number of other countries reducing the market dominance of these ten countries. Among the ten countries with the most RNo registered, China has a high increase in the number of RNo. In the period 2000 – 2006, China was ranked 12th among the countries with the most RNo in Vietnam (accounting for 1,81%), but between 2009 and 2019, China accounted for 3,5% and ranked 4th among the ten countries with the most RNo, higher than France (with 3,3%), which was the third country in the period of 2000 – 2006, accounting for 7,0% of the total RNo
The classification of drugs in single dosage forms and combination drugs from 2009 through 2019 accounted for about 80,0% with 34.055 RNo, drugs in combination with 8.502 RNo (accounting for 20,0%). In particular, the domestic single dosage form drug ratio structure is lower than the ratio structure of the foreign single-component drug (the average domestic single-component drug accounts for 77,8% of the total domestic drugs, and this ratio is 83,3% for foreign drugs). The percentage of single dosage forms has increased significantly in recent years; it only accounted for 55 – 65% of the total modern medicines’ RNo in 2001 – 2005 and 70,8 – 85,6% in 2009 – 2019. This trend shows consistency with the recommendations of the MOH when prioritizing the use of a single dosage form. In the case of combination drugs, it must be demonstrated that the combination is more beneficial when the individual ingredients are used in terms of efficacy and safety. Herbal and traditional drugs were mainly in the combination form, accounting for 80,6%, about four times as much as the single dosage form (19,4%), because most herbal and traditional drugs are generally based on ancient remedies, which are a combination of ingredients according to the theory and method of traditional medicine. For vaccines, the individual vaccines account for 74.8% of the total 110 RNo, almost three times more than the combination vaccine (37 RNo with 25.2%). For domestic combination vaccines, 8 RNo were issued, including two types of 2-in-1 combination vaccines (i.e., diphtheria, tetanus, measles, rubella) and a 3-in-1 combination vaccine (i.e., diphtheria, pertussis, tetanus).

Tablets are the most registered dosage form in both domestic and foreign drugs, accounting for about 46,4%. In particular, capsules are the second most commonly used formulation in Viet Nam (accounting for 18,6%) and the majority come from domestically manufactured drugs. In the case of injectable drugs, 16.9% were mainly drugs manufactured by foreign countries. Paracetamol is the most registered active ingredient, mainly in domestically manufactured drugs (accounting for 2068/2260 RNo). In particular, vitamins such as vitamins B1, B6, C, and other vitamins have a large number of RNo but most of them are in the form of a combination of vitamins and minerals and do not have many specific pharmacological effects. However, from 2014 to 2019, the number of RNo granted to vitamins has tended to decrease, which is reflected in the fact that most vitamins are no longer included in the list of the 20 most registered active substances. For vitamins still on the list, the ranking tends to decrease compared to previous years. Protamine is an active ingredient on the list of essential drugs of both WHO and MOH, but no RNo is registered in our country. Currently, this active ingredient is still being imported without registration. Therefore, pharmaceutical enterprises need to prioritize research and development, production with the drugs having few RNo or no RNo or expired RNo, specifically 508 active ingredients issued in Decision No. 11599/QLD-DK dated June 30, 2015, of the Drug Administration.
One of the most serious difficulties when researching the implementation of this topic is that most of the information on the list of drugs granted registration numbers posted on the Department of Health Management (dav.gov.vn) is not complete. For the information of the drugs in the list of registration numbers, there is still a lack of information such as content, name, and recording of the unidentified active ingredients, leading to difficulties in the data processing. And the homogenization of the names and inscriptions of the active substances in the current list of registration numbers has not yet been specified in any legal documents in our country. Therefore, in the future, to develop other studies on this issue to be more effective, the State management agency needs to regularly publish and update the list of RNo on the website; there are specific regulations on the uniformity of names and names of active ingredients in the lists of RNo. The overview of the situation of medicine registration in Viet Nam from 2009 to 2019 helps Pharmaceutical Management Government agencies, and enterprises (domestic and foreign) to grasp the tendencies and needs of the Vietnam pharmaceutical market. This is the basis to orientate the development of the pharmaceutical market and propose appropriate healthcare policies.
Ministry of health (1989), “Decision no. 249/BYT-QD Promulgating the regulation on registration of domestic drugs and registration of foreign drugs in Vietnam”.
Ministry of Health (1996), “Decision No. 1203/BYT-QD Promulgating Regulations on Medicine Registration “.
Ministry of Health (2001), “Decision No. 3121/2001/QD-BYT on registration of drugs “.
Ministry of Health (1996), “Decision No. 2010/BYT-QĐ Regulations on registration of vaccines and immune bio-products”.
Ministry of Health (2009), “Circular No. 22/2009/TT-BYT Drug Registration Regulation “
Ministry of Health (2003), “Circular No. 4012/2003/QĐ-BYT Promulgating regulations on registration of vaccines, medical biologicals”
Ministry of Health (2004), “Decision No. 3947/2004/QĐ-BYT Promulgating regulations on registration of vaccines, medical biologicals”
Ministry of Health (2009), “Circular No. 22/2009/TT-BYT Drug Registration Regulation”
Ministry of Health (2014), “Circular No. 44/2014/TT-BYT Drug Registration Regulation”
Drug Administration, “New points in drug registration Circular 44/2014/TT-BYT”
Ministry of Health (2018), “Circular No. 32/2018/TT-BYT of the Ministry of Health on marketing authorization of drugs and medicinal ingredients ” .
Van B. T. (2006), Initially analyzing and evaluating the group of foreign drugs signed by the party for circulation in Vietnam, Department of Pharmaceutical Economics and Management, Hanoi University of Pharmacy.
Ministry of health (2018), “Circular No.19/2018/TT-BYT introducing list of essential medicines”.
Source: Article based on the publication from the collaborative author: Nguyen Thi Hai Yen, Thai Hue Ngan, et al. at Ho Chi Minh City University of Medicine and Pharmacy. The data are cited with the consent of the collaborative author. (https://js.vnu.edu.vn/MPS/article/view/4297)





You must be logged in to post a comment.