ORIOLED HUB INSTITUTE OF PHARMACEUTICAL TECHNOLOGIES
The Institute of Pharmaceutical Technology was established by Orioled Hub company and recognized by the Department of Science and Technology of Ho Chi Minh City in August 2022. Headquarters: 72 Binh Gia Street, Ward 13, Tan Binh District, Ho Chi Minh City. Director: Mr. Pham Ngoc Vien Linh Senior Advisor: Dr. Le Minh Quan, PhD
- Published in blog
Classification of functional foods being circulated on the market in Vietnam
The below contents do not contain the full and detailed information of the circular, but the information is extracted and summarized as a part of the circular on classifying functional foods including supplemented foods, health protection foods, medical foods, and foods used for special dietary uses. Circular 43 classifies functional foods into 4 main groups
- Published in blog
What is a Sunset Clause?
Before any product can be sold within the European market, it needs a market authorization (MA) from European Medicines Agency (EMA). Once a medicinal product gets MA from the European Medicines Agency (EMA), it is ready to be sold in EU. However, the Marketing Authorization Holder (MAH) must follow some mandates: The medicinal product must
- Published in blog
Happy Birthday TV TPI
Today, TPI TV has turned 7 years old, the process is not too short nor long but enough for TPI TV to gradually mature and become more stable. Over the past 7 years, the number of members in the TPI TV family is increasing day by day, people come and go, but they all contribute to making TPI TV stronger and constantly innovating as it is now.
- Published in event
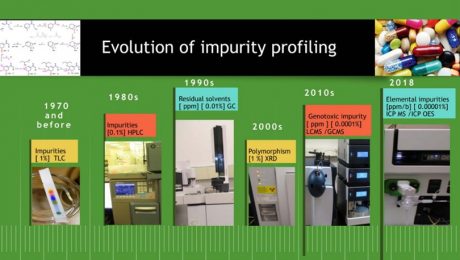
Impurity Profiling of Solid Oral Drug Products
1. Introduction Impurity is considered as any component of a drug substance that is not a chemical entity defined as the drug substance and in addition, for a drug product, any component that is not a formulation ingredient [1]. Impurity profiling is the basis for determining, assuring the quality, safety, and efficacy of drug substances
- Published in blog
EFFECTIVE PLANNING TOOL
Planning is one of the necessary skills in both work and life if you want to handle work effectively, and systematically, increase initiative, and control as well as easily cope if faced with a difficult situation and deal with the risks. Planning is not only good for you personally but is especially useful in an
- Published in blog
[ORIOLED HUB TRADE UNION] – JUNE IS FAMILY MONTH
In June, in addition to “International Children’s Day” (June 01) which is an occasion to show affection to the preschool children of each family, we have many other anniversaries: “Vietnam Family Day 28/06”. as a loving reminder of the care and affection of the members of each Vietnamese family home, World Day against Child Labor
- Published in event
On bidding for supply of drugs for public health facilities (or how to classify tender groups of medicines to be reimbursed by public health insurance in VN)
The following below contents do not contain the full and detailed information of the circular, but the information is extracted and summarized briefly as a part of the circular on chemical drugs and biological products in the public tender system. Besides a separated tender group for the original branded products (or the originator), there are
Overview of drug registration in Vietnam in the period 2009-2019
Since 2008, Vietnam has been classified as a pharmerging country, according to IMS Health (IQVIA). Pharmaceutical spending per capita in Viet Nam is relatively low but has increased dramatically in recent years. In 2005: 9,85 USD (equivalent to about 227.000 VND), in 2014: 34,48 USD (equivalent to about 793.000 VND), forecasted to reach to 163
- Published in blog
The approval process of medicines in Europe
The European system of approval of new medicines comprises an European Union (EU)-wide authorisation procedure (the so called centralised pro- cedure) alongside national procedures based on different EU Member States working together and recognizing each other’s evaluations (the so-called decentralised and mutual recognition procedures). It is a system that has evolved over the past half-century
- Published in blog