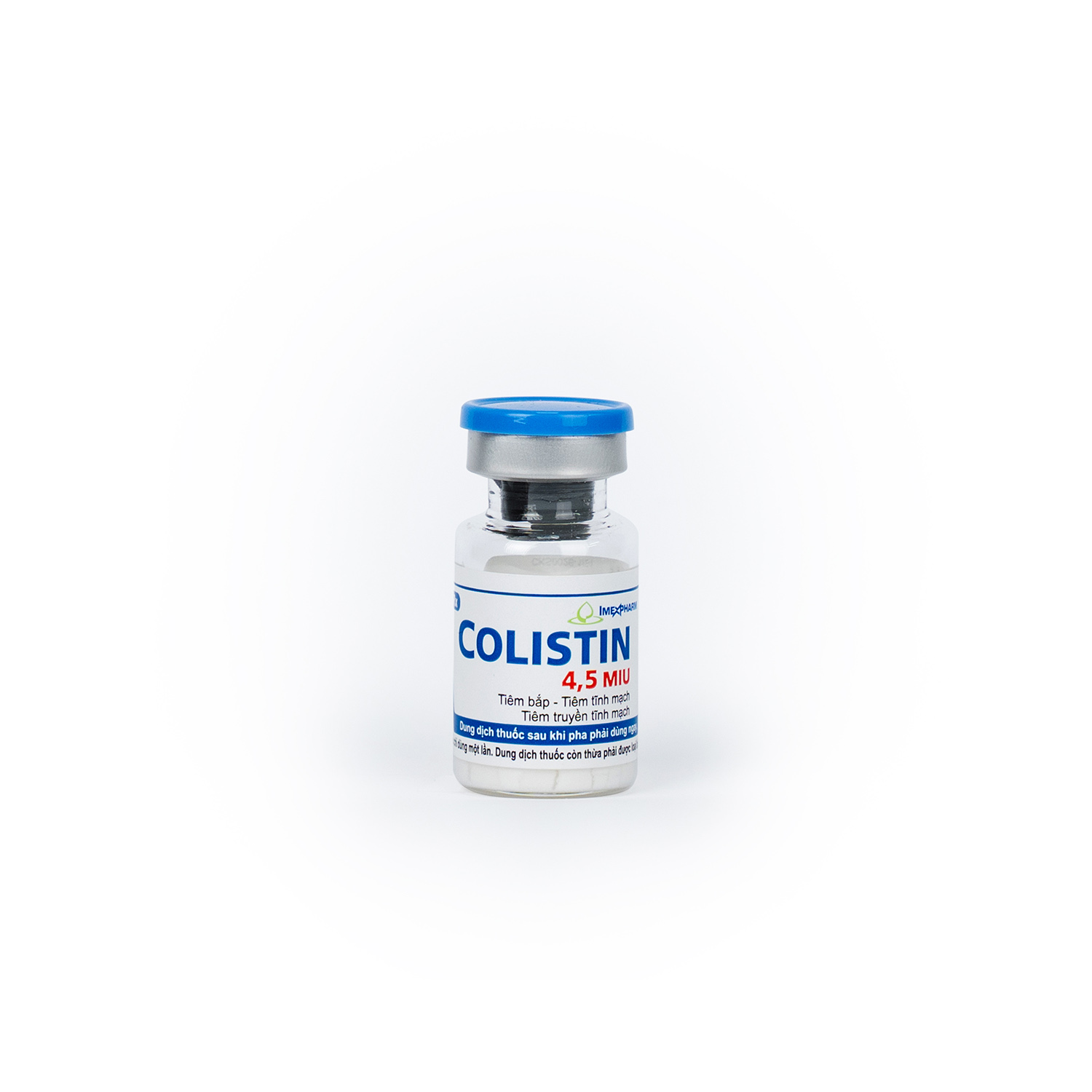

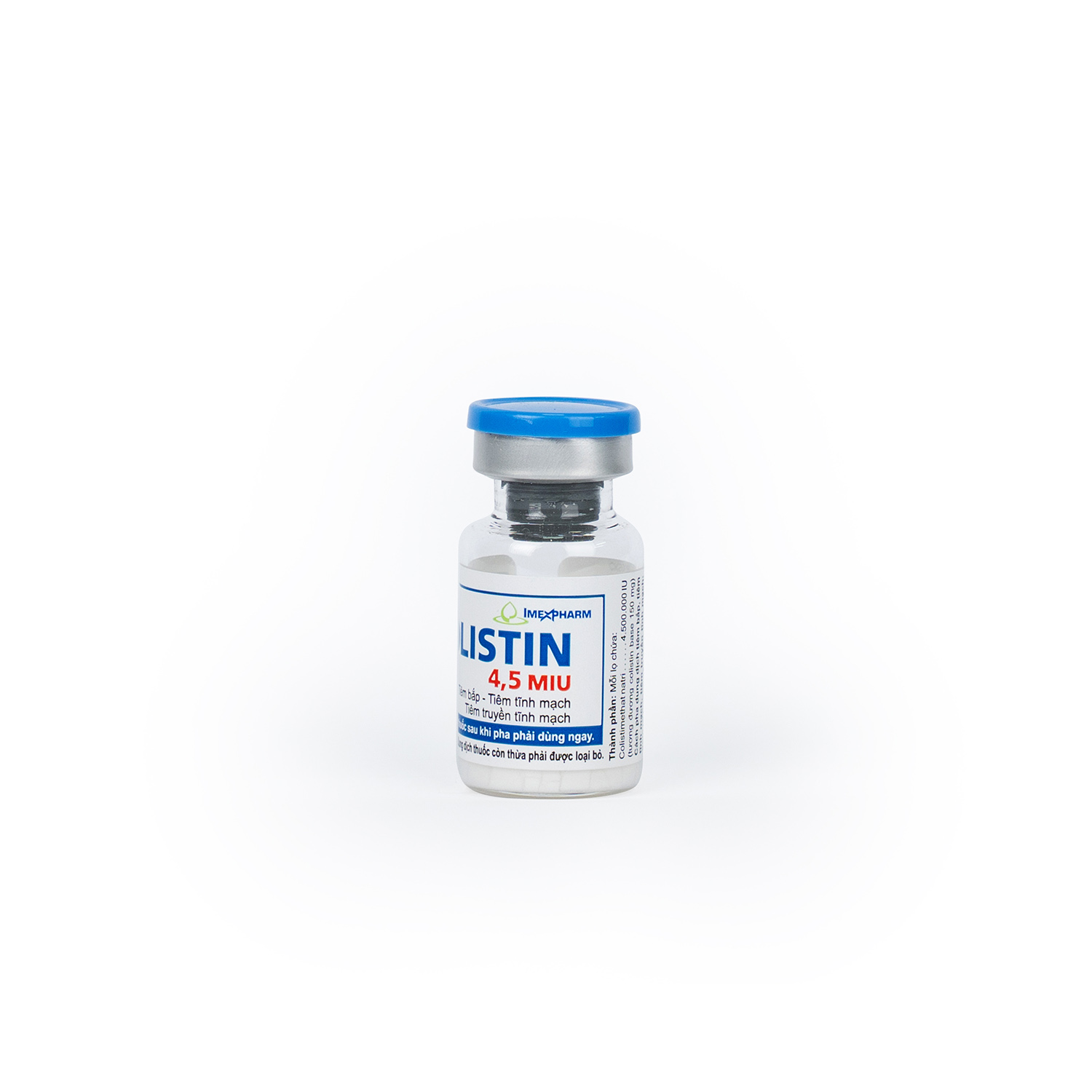
Colistin 4,5 MIU
Manufacturer
Branch of Imexpharm Corporation - Binh Duong Hi-Tech Plant
Applicant company
Imexpharm Pharmaceutical Joint Stock Company
Active ingredient
Colistimethate sodium (equivalent to 150 mg colistin base activity)
Content
4.500.000 IU
Shelf life
24 months
Dosage Form
Lyophilized powder for injection
Package
Box of 1 vial, 5 vials, 10 vials
Visa Number
893114229224
Issuing Date
21/03/2024
Therapeutic area
Antibiotics
Indication
Colistin 4.5 MIU is indicated when other drugs cannot be used to treat severe infections caused by Gram-negative bacteria: Sepsis, meningitis, kidney infections, urinary tract infections - genitals.
Dosage
Adults and children with normal renal function
The recommended dose is 2,5 – 5 mg colistin base/kg/day, divided into 2 – 4 times/day depending on the severity of the infection.
Renal impairment
The dose and frequency of use should be reduced in patients with renal impairment. The dosage regimen is adjusted according to the following recommendations:
| Plasma creatinine concentration (mg/dL) | Dosing regimen |
|---|---|
| 1,3 – 1,5 | 75 - 115 mg colistin base/time, 2 times/day (equivalent to a dose of 2.5 - 3.8 mg colistin base/kg/day) |
| 1,6 – 2,5 | 66 - 150 mg colistin base/time, 1 - 2 times/day (equivalent to a dose of 2.5 mg colistin base/kg/day) |
| 2,6 – 4 | 100 - 150 mg colistin base/time, every 36 hours (equivalent to a dose of 1.5 mg colistin base/kg/time) |
Contact