
QUIMOX
Manufacturer
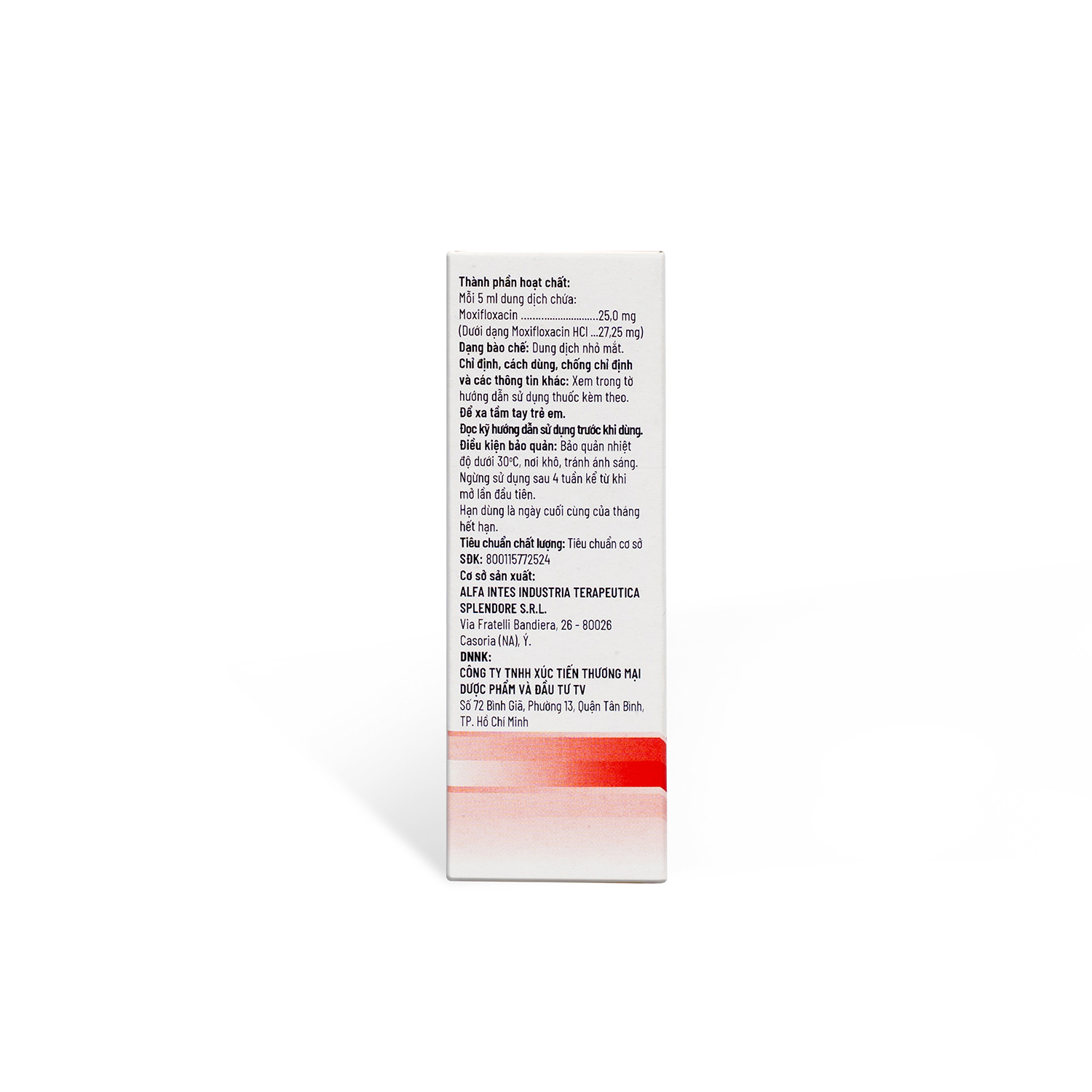
ALFA INTES INDUSTRIA TERAPEUTICA SPLENDORE S.R.L., Italy
Applicant company
TV TPI CO. LTD.
Active ingredient
Moxifloxacin
Content
25mg
Shelf life
24 months
Dosage Form
Ophthalmic solution
Package
Box of 1 vial x 5 ml
Visa Number
800115772524
Issuing Date
12/8/2024
Therapeutic area
Ophthalmologicals; anti-infectives, other anti-infectives
Indication
Quimox is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following microorganisms:
Aerobic Gram-positive microorganisms:
Corynebacterium species
Microbacterium species
Micrococcus luteus (including strains resistant to erythromycin, gentamycin, tetracycline and/or trimethoprim).
Staphylococcus aureus (including strains resistant to methicillin, erythromycin, gentamycin, ofloxacin, tetracycline and/or trimethoprim).
Staphylococcus epidermidis (including strains resistant to methicillin, erythromycin, gentamycin, ofloxacin, tetracycline and/or trimethoprim).
Staphylococcus haemolyticus (including strains resistant to methicillin, erythromycin, gentamycin, ofloxacin, tetracycline and/or trimethoprim).
Staphylococcus hominis (including strains resistant to methicillin, erythromycin, gentamycin, ofloxacin, tetracycline, and/or trimethoprim).
Staphylococcus warneri (including strains resistant to erythromycin).
Streptococcus pneumoniae (including strains resistant to penicillin, erythromycin, tetracycline, and/or trimethoprim).
Stretococcus viridans (including strains resistant to penicillin, erythromycin, tetracycline, and/or trimethoprim).
Aerobic Gram-negative microorganisms:
Acinetobacter species
Haemophilus alconae (including ampicillin-resistant strains).
Haemophilus influenzae (including ampicillin-resistant strains).
Haemophilus parainfluenzae
Klensialla pneumoniae
Moraxella cattarrhalis
Pseudomonas aeruginosa.
Other microorganisms:
Chlammydia trachomatis
Effectiveness on this microorganism has been studied in less than 10 infections.
In addition, Quimox eye drops are used in the following cases:
- Treatment of corneal ulcers.
- Use before and after surgery to prevent infection.
Dosage
Adults and elderly (≥ 65 years)
Insert 1 drop into the affected eye 3 times/day for 7 days. The inflammation usually improves after 5 days, treatment should be continued for 2-3 more days. If the inflammation does not improve after 5 days of treatment, the diagnosis and/or treatment should be reconsidered.
Children
No dosage adjustment is required.
People with liver and kidney failure
No dosage adjustment is required.
Contact