Impurity Profiling of Solid Oral Drug Products
Thursday, 07 July 2022
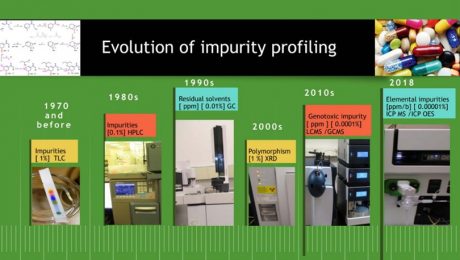
1. Introduction Impurity is considered as any component of a drug substance that is not a chemical entity defined as the drug substance and in addition, for a drug product, any component that is not a formulation ingredient [1]. Impurity profiling is the basis for determining, assuring the quality, safety, and efficacy of drug substances
- Published in blog