GROUNDBREAKING CEREMONY FOR REIWA PHARMACEUTICAL FACTORY – REALIZING THE GOAL OF CONQUERING A NEW VISION
On October 1st, 2024, Reiwa Pharmaceutical Enterprise (Reiwapharm) officially broke ground at Dat Do 1 Industrial Park, Phuoc Long Tho Commune, Dat Do District, Ba Ria – Vung Tau Province. This marks a new milestone, opening up a journey to conquer strategic goals, contributing to completing the comprehensive pharmaceutical supply chain. With a total floor
- Published in event
ORIOLED HUB INSTITUTE OF PHARMACEUTICAL TECHNOLOGY SIGNS A COOPERATION AGREEMENT WITH THONG NHAT HOSPITAL TO ESTABLISH A BIOEQUIVALENCE AND CLINICAL TRIALS CENTER
On August 22, 2024, Orioled Hub Institute of Pharmaceutical Technologies (OHI) and Thong Nhat Hospital officially signed a cooperation agreement to establish a Bioequivalence and Clinical Trials Center. This strategic partnership marks a significant milestone in the Vietnamese pharmaceutical industry, promising to accelerate clinical trials and enhance the global acceptance of bioequivalence study results, thereby
- Published in event
Orioled Hub is honored to be the Silver sponsor of the 39th Pharmaceutical Science and Technology Conference of Ho Chi Minh City University of Medicine and Pharmacy
On April 6-7, Orioled Hub held a small booth at the 39th Science and Technology Conference of the University of Medicine and Pharmacy in Ho Chi Minh City. HCM. In the hope of opening more opportunities to meet and interact with pharmacists from all over the world, with many exciting activities and thousands of attractive
- Published in event
Summary of intraocular lens market information in Vietnam 2021-2022
The following article provides information, overview data and latest trends about the intraocular lens market imported to Vietnam. The data provided in the article is based on Big data of Orioledhub. A. MARKET OVERVIEW An Intraocular Lens implant (or artificial lens implant) is a synthetic lens placed inside the eye that replaces the focusing power
- Published in blog
ORIOLEDHUB’S TELLER: MEMORY
On August 11, 2022, after more than a month of practice, the final show of the emotional Orioledhub’s Teller: Memory program officially took place. Each of us from birth has been a particular version of ourselves, there is only one on this earth, but sometimes the prejudices, the thoughts that force us to be like
- Published in event
ORIOLED HUB INSTITUTE OF PHARMACEUTICAL TECHNOLOGIES
The Institute of Pharmaceutical Technology was established by Orioled Hub company and recognized by the Department of Science and Technology of Ho Chi Minh City in August 2022. Headquarters: 72 Binh Gia Street, Ward 13, Tan Binh District, Ho Chi Minh City. Director: Mr. Pham Ngoc Vien Linh Senior Advisor: Dr. Le Minh Quan, PhD
- Published in blog
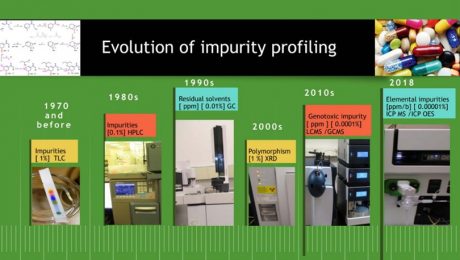
Impurity Profiling of Solid Oral Drug Products
1. Introduction Impurity is considered as any component of a drug substance that is not a chemical entity defined as the drug substance and in addition, for a drug product, any component that is not a formulation ingredient [1]. Impurity profiling is the basis for determining, assuring the quality, safety, and efficacy of drug substances
- Published in blog