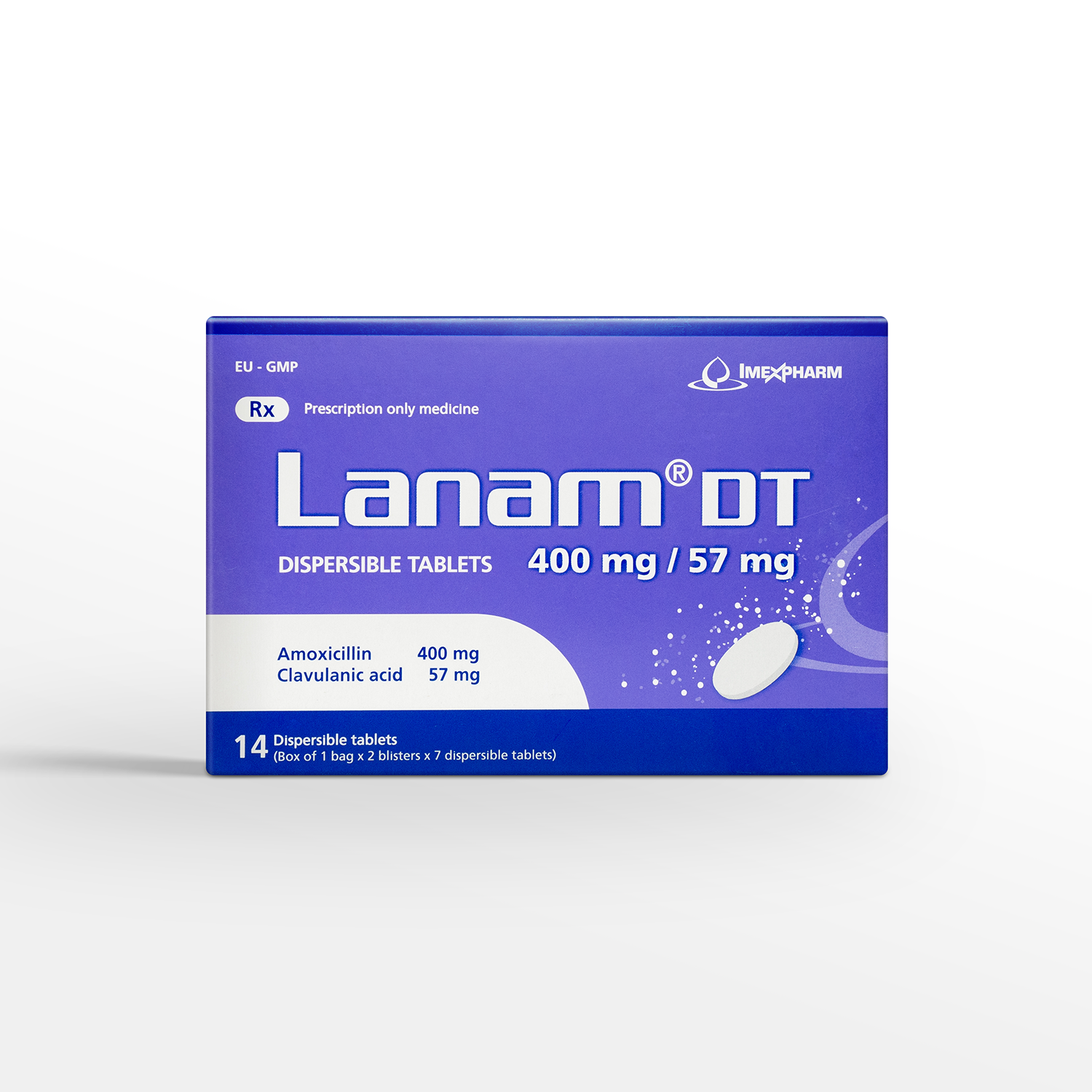




Lanam DT 400mg/57mg
Manufacturer
Branch of Imexpharm Corporation - Vinh Loc Hi-Tech Pharmacetical Antibiotic Plant
Applicant company
Imexpharm Pharmaceutical Joint Stock Company
Active ingredient
Amoxicilin (as Amoxicilin trihydrat compacted), Acid Clavulanic (as Kali Clavulanat - Avicel (1:1))
Content
400 mg / 57 mg
Shelf life
24 months
Dosage Form
Dispersible tablet
Package
Box of 1 bag x 2 blisters x 7 dispersible tablets
Visa Number
893110848124
Issuing Date
23/08/2024
Therapeutic area
Antibiotics
Indication
The drug is indicated in the treatment of infections caused by bacteria sensitive to the drug such as:
- Acute bacterial sinusitis.
- Acute otitis media.
- Acute exacerbation of chronic bronchitis.
- Community-acquired pneumonia.
- Cystitis.
- Pyelonephritis.
- Skin and subcutaneous tissue infections, especially in cellulitis, insect bites, severe dental abscesses leading to cellulitis.
- Bone and joint infections, especially osteomyelitis.
Dosage
Dosages are usually expressed in terms of amoxicillin/clavulanic acid unless otherwise stated in terms of the individual components.
Dosage selection of Lanam DT 400 mg/ 57 mg depends on the following factors:
- Type of pathogenic bacteria and sensitivity to antibacterial agents.
- The severity and site of the infection.
- The age, weight and renal function of the patient.
The use of alternative medicines to Lanam DT 400 mg/57 mg (e.g. when higher doses of amoxicillin and/or different ratios of amoxicillin to clavulanic acid are required) should be considered when necessary.
For children weighing <40 kg, when used as recommended, the Lanam DT 400 mg/57 mg formulation provides a maximum daily dose of 1,000 – 2,800 mg amoxicillin/143 – 400 mg clavulanic acid. If a higher daily dose of amoxicillin is required, a different amoxicillin/clavulanic acid combination should be selected to avoid unnecessary high doses of clavulanic acid.
Treatment duration should be considered according to the patient's response. Some infections (e.g. osteomyelitis) require longer treatment. Treatment should not be extended beyond 14 days without reassessment of the patient's condition.
Adults and children weighing 40 kg or more should use a product with a more appropriate strength of amoxicillin/clavulanic acid.
Children weighing < 40kg:
- Recommended dose: (calculated per kg of weight)
• 25 mg/3.6 mg/kg/day to 45 mg/6.4 mg/kg/day, divided into two oral doses.
• An increase in dose up to 70 mg/10 mg/kg/day given in two divided doses may be considered for some infections (e.g. otitis media, sinusitis, lower respiratory tract infections).
There are no clinical data on the use of amoxicillin/clavulanic acid 7:1 at doses above 45 mg/6.4 mg/kg/day in children under 2 years of age. There are no clinical data on the use of amoxicillin/clavulanic acid 7:1 in children under 2 months of age. Therefore, dose recommendations for these subjects have not yet been made.
- Or the recommended dose is calculated according to the dosage form:
Weight
15 kg to under 30 kg: 1 tablet/time, 2 times/day. The dose can be increased to 2 tablets/time, 2 times/day
30 kg to under 40 kg: 1 - 2 tablets/time, 2 times/day. Dosage can be increased to 3 tablets at a time, 2 times a day
Elderly people
Dosage adjustment is not necessary.
Patients with renal failure:
Patients with creatinine clearance (CrCI) greater than 30 mL/min: no dose adjustment is required.
Patients with creatinine clearance less than 30 mL/min: the combination of amoxicillin/clavulanic acid at a ratio of 7:1 is not recommended, therefore, there is no data on dose adjustment.
Patients with liver failure: use with caution and periodically check liver function during use of the drug.
Contact