
1. Introduction
Mean Kinetic Temperature (MKT) is used in the pharmaceutical industry to evaluate the effect fluctuating temperatures have over time on the efficacy and safety of a drug product. In 1971, J.D. Haynes calculated a “Virtual Temperature” to predict product expiration when considering temperature variability in a given region. The formula uses the Arrhenius equation, which identifies the temperature dependence on chemical reaction rates. The higher (and longer) a drug product is exposed to high temperatures, the faster it decomposes.
The U.S. Food and Drug Administration (FDA) and European Commission recognizes MKT as a mathematical tool to help identify safe shipping and storage conditions within specific climatic zones.
2. Storage conditions
| | Ph. Eur | USP |
| Frozen | -150C | -25 to -100C (freezer) |
| Fridge | 2-80C | 2-80C (cold) |
| Cold | 8-150C | 8-150C (cool) |
| Room temperature | 15-250C | Not defined |
| Controlled room temperature | - | 20-250C (in exceptional cases: 15-300C -> related to MKT) |
3. Definition
MKT is the single calculated temperature at which the total amount of degradation over a particular period is equal to the sum of the individual degradations that would occur at various temperatures. MKT may be considered as an isothermal storage temperature that simulates the non-isothermal effects of storage temperature variation. It is not a simple arithmetic mean.
4. When is MKT used?
Stability Study: MKT can be used to simulate (predict) the decomposition of a drug substance during a stability study.
Storage: In regulatory documents, there is a wide consensus that MKT can be used to assess temperature excursions outside specified storage conditions of refrigerated and room temperature products.
Transport: Some suggest using MKT in transport environments.
5. Calculation of MKT
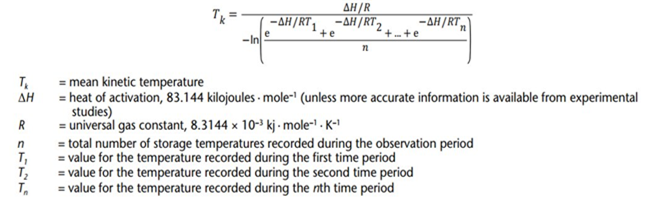
Nowadays, we have many tools replacing manual calculation to calculate MKT value such as MS Excel, DicksonOne Software, Softpedia Software, …
6. MKT regulations in United States Pharmacopeia (USP)
MKT has been misunderstood and/or misused. The most significant misuse has been utilizing 52 weeks of temperature data to calculate MKT during a temperature excursion. Drug products typically do not spend 52 weeks in a single storage location. Thus, the 52 weeks of data used in the MKT calculation would not be a true reflection of the storage time. This approach skews results and could lead a company to overlook the impact of an excursion on the drug product. A closely related concern is the idea that a temperature excursion above a product’s storage temperature can be “fixed” just by lowering the temperature of a warehouse for an appropriate period of time so that the resulting MKT calculation would provide an acceptable value. This ignores the fact that any degradation due to the higher temperature is not reversible.
MKT is referenced in the controlled room temperature (CRT) and controlled cold temperature (CCT) definitions in Packaging and Storage Requirements <monograph 659>. Within the definitions, the temperature range for an excursion and the maximum excursion time are defined, but the time frame used for calculating MKT is not mentioned. For CRT excursions, USP recommends using 30 days for calculating MKT or the average number of days that a product remains in the holder’s possession. This recommendation is based on temperature data presented by Anderson et al and the fact that products, on average, spend 30 days in warehouse storage in the United States.
For CCT excursions, USP recommends using 24 h for calculating MKT. While specific products may have stability data to support excursions within wider temperature and time frames, operations involved with storage and distribution (shipping) throughout the supply chain typically do not have access to information beyond the product labeling. This includes dispensing and healthcare providers. Excursions occur and these guidelines are intended to manage these excursions while being conscious of the impact of temperature and time excursions. It is important to note that an excursion is a nonconforming event (except as previously noted when provided by the manufacturer). MKT should not be used to justify a system out of control (e.g., repeated temperature and time excursions).
| Storage Range | Acceptable Excursion Range | Maximum Temperature | Maximum Excursion Time (NMT) | MKT (NMT) | Time period of Calculation | |
| CRT | 200 – 250 | 150 – 300 | 400 | 24h | 250 | 30 days |
| CCT | 20 – 80 | 80 – 150 | 150 | 24h | 80 | 24h |
In CRT storage environments, temperature monitoring captures the temperatures throughout the day (e.g., every 15 min). MKT can be calculated on an ongoing basis or anytime that there has been a temperature excursion using data going back 30 days from (and including) the high excursion temperature. 30 days must be from, and including, the high excursion temperature for the entire period of the excursion.




