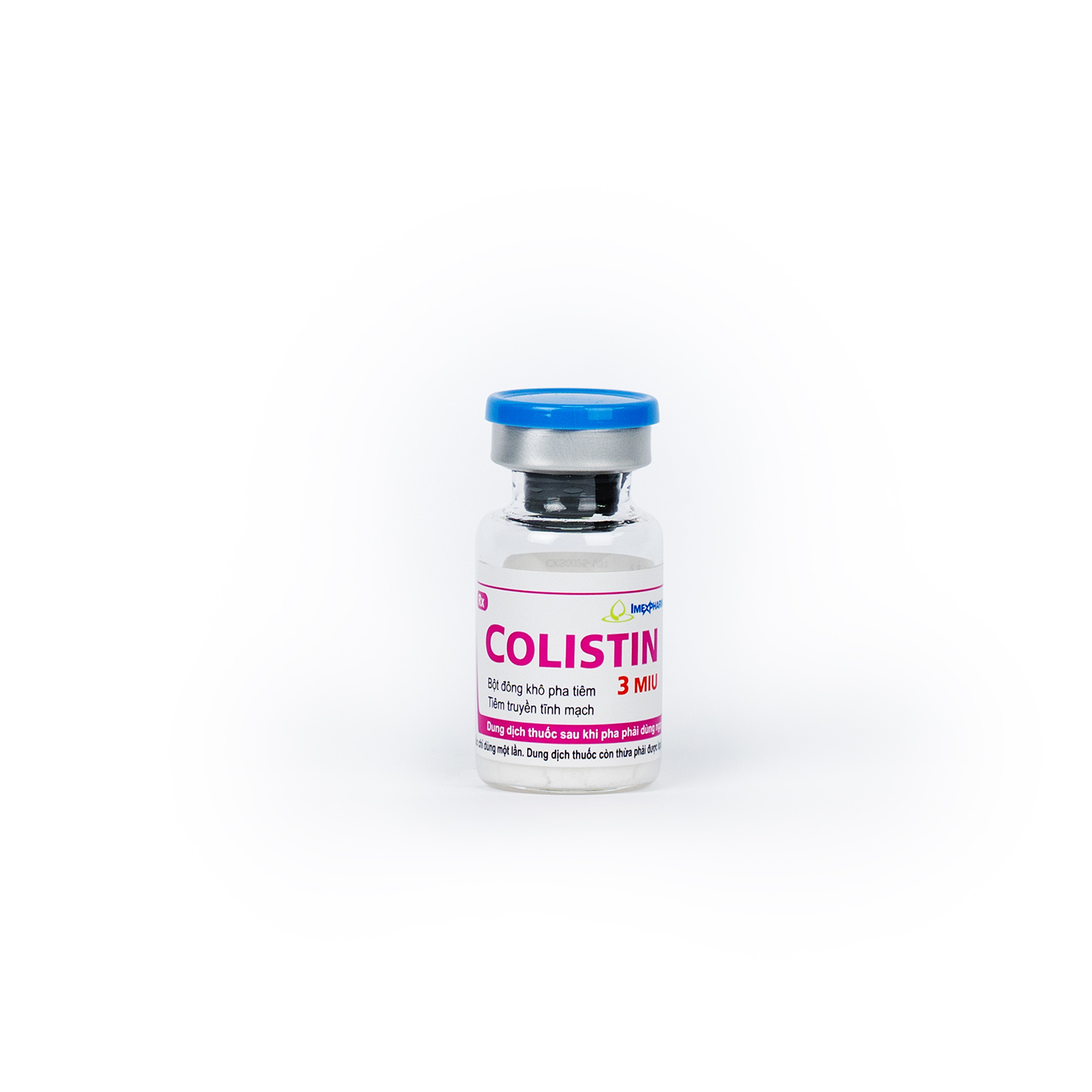




Colistin 3MIU
Manufacturer
Branch of Imexpharm Corporation - Binh Duong Hi-Tech Plant
Applicant company
Imexpharm Pharmaceutical Joint Stock Company
Active ingredient
Colistimethate sodium (equivalent to colistin base 100mg)
Content
3.000.000 IU
Shelf life
24 months
Dosage Form
Lyophilized powder for solution for injection
Package
Box of 1 vial, Box of 5 vials, Box of 10 vials
Visa Number
893114940624
Issuing Date
16/09/2024
Therapeutic area
Antibiotics
Indication
Colistin 3 MIU is indicated for the treatment of severe infections caused by selected aerobic Gram-negative bacteria in patients with limited treatment options: sepsis, meningitis, kidney infections, and urinary-genital tract infections (see sections on Dosage and Administration, Warnings and Precautions, and Pharmacodynamics).
Dosage
The dosage and duration of treatment should be based on the severity of the infection as well as the clinical response. Treatment guidelines should be followed. The dosage is expressed in IU (International Units) of colistimethate sodium (CMS). The conversion table from CMS units “IU” to “mg” and the corresponding conversion to “mg” of colistin base (CBA) are listed in the Administration section. The following dosage recommendations are based on pharmacokinetic data from a number of critically ill patients (see Warnings and Precautions section).
Adults and Adolescents
The maintenance dose is 9 MIU/day, divided into 2-3 doses. In critically ill patients, an initial dose of 9 MIU should be used. The optimal timing for the first maintenance dose has not yet been determined.
Modeling suggests that an initial dose and maintenance dose of up to 12 MIU may be required in patients with normal kidney function in certain cases. However, clinical experience with such dosing is very limited, and safety has not been established.
The initial dose applies to patients with normal kidney function, impaired kidney function, and those undergoing renal replacement therapy.
Renal Impairment
Dosage adjustments are necessary for patients with renal impairment. However, pharmacokinetic data in patients with renal impairment are very limited. Therefore, the suggested dose adjustments are as follows:
| Creatinine clearance (mL/min) | Daily dosage |
|---|---|
| < 50 – 30 | 5,5 – 7,5 MIU, divided into 2 times/ day. |
| < 30 – 10 | 4,5 – 5,5 MIU, divided into 2 times/ day. |
| <10 | 3,5 MIU, divided into 2 times/ day. |
Hemodialysis and Continuous Renal Replacement Therapy
Colistin can be removed through conventional hemodialysis and continuous veno-venous hemofiltration or hemodiafiltration (CVVHF, CVVHDF). Pharmacokinetic data in a small number of patients undergoing renal replacement therapy remain limited. Therefore, fixed dosing is not recommended, but the following regimens may be used for reference:
- Hemodialysis:
Non-dialysis days: 2.25 MIU/day (range: 2.2–2.3 MIU/day)
Dialysis days: 3 MIU/day, administered post-dialysis
The total daily dose should be divided into two administrations.
- Continuous veno-venous hemofiltration/hemodiafiltration (CVVHF, CVVHDF):
Dosage is similar to that in patients with normal renal function.
It is recommended to divide the total daily dose into three administrations.
Hepatic Impairment:
There are no data available in patients with hepatic impairment. Colistimethate sodium should be used with caution in these patients.
Elderly:
No dose adjustment is necessary in elderly patients with normal renal function.
Pediatrics:
Data to support accurate dosing in pediatric populations are very limited. Renal maturity should be taken into account when selecting a dose. Dosing should be weight-based.
Children ≤ 40 kg: 75,000 – 150,000 IU/kg/day, divided into three doses.
For children weighing over 40 kg, the adult recommended dose should be considered.
Doses >150,000 IU/kg/day have been reported in children with cystic fibrosis.
No data are available on loading doses in critically ill pediatric patients.
No recommended dosing regimen has been established for children with renal impairment.
In patients requiring dose reduction (such as those with moderate to severe renal impairment or children ≤ 40 kg), colistimethate sodium 1 MIU or 2 MIU formulations should be used in appropriate doses.
Contact