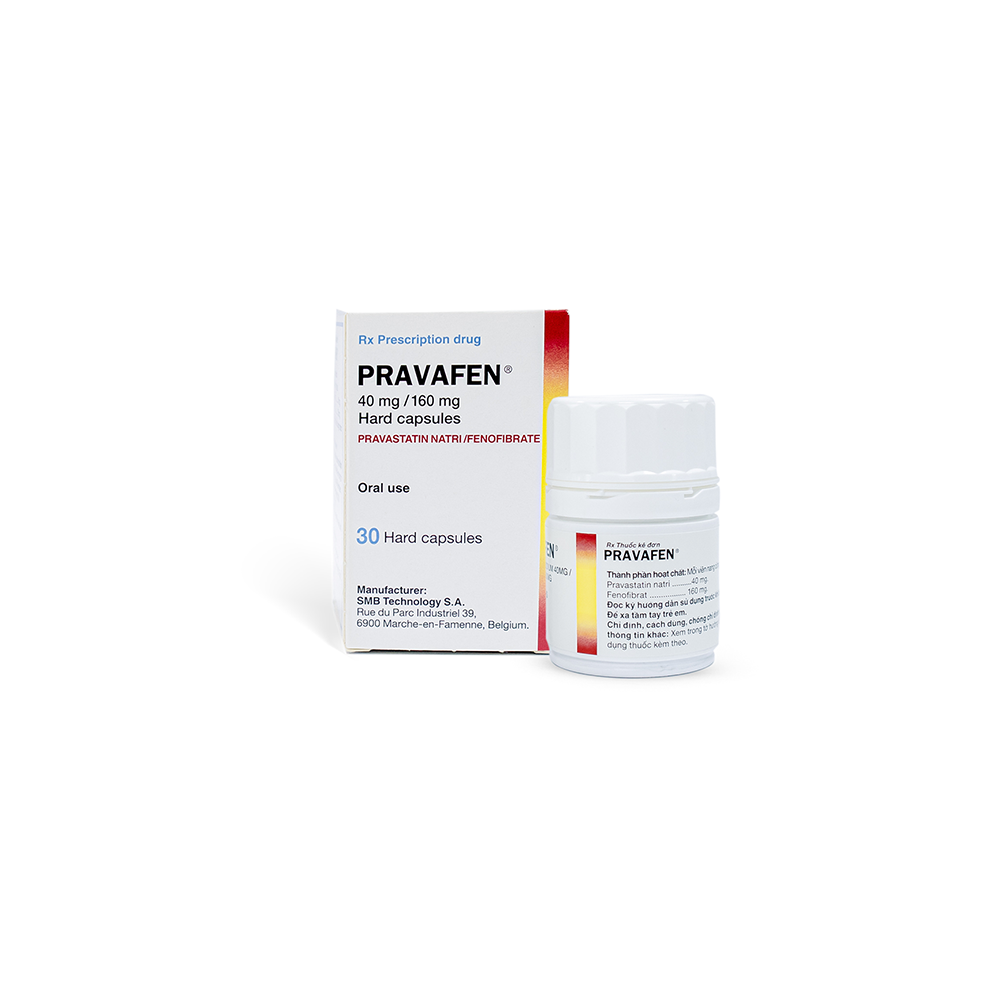




PRAVAFEN
Therapeutic area
Diabetes & Hyperlipidemia
Manufacturer
SMB Technology S.A, Belgium
Visa Number
540110196923 (VN3-156-19)
Issuing date
14/07/2023
Applicant company
TV TPI CO. LTD.
Active ingredient
Pravastatin sodium, Fenofibrate
Content
40 mg, 160 mg
Indication
Pravafen is indicated for use in combination with diet and other non-pharmacological treatments (e.g. exercise, weight loss) for the treatment of mixed hyperlipidemia in at-risk adult patients. cardiovascular disease to reduce triglycerides and increase HDL-C when LDL-C concentrations are adequately controlled during treatment with pravastatin 40 mg monotherapy.
Dosage
Recommended dose is 1 tablet/day. Patients should continue to maintain the dietary restrictions established before starting treatment.
Treatment response should be monitored by measuring serum lipid index. Serum lipid concentrations usually decrease rapidly during treatment with Pravafen; if an adequate response is not achieved within three months of treatment, the drug should be discontinued.
Use of drugs in special patient groups:
Elderly people (≥ 65 years old):
Treatment with Pravafen should be initiated after assessment of renal function (see Renal and urinary disorders).
Safety data for Pravafen in patients over 75 years of age are limited, therefore caution should be exercised when administering the drug to these patients.
Patients with kidney failure:
Pravafen is contraindicated in patients with severe and moderate renal impairment (when creatinine clearance < 60 ml/min, see Contraindications).
No dosage change is necessary for patients with mild renal impairment.
Patients with liver failure:
Pravafen is not recommended for use in patients with moderate hepatic impairment and is contraindicated in patients with severe hepatic impairment (see Contraindications). No dose adjustment is necessary in patients with mild hepatic impairment.
Children (< 18 years old):
Pravafen is not suitable for use in children for the treatment of mixed dyslipidemia (see Contraindications).
Shelf life
36
Dosage Form
Hard capsule
Package
Box of 1 bottle x 30 capsules
Contact